Abstract
Bromodomain and extraterminal domain (BET) proteins play a key role in the regulation of neoplastic myeloproliferation and proinflammatory cytokine production in myelofibrosis (MF). Pelabresib (CPI-0610; PELA) is an oral, small-molecule, investigational BET inhibitor that downregulates NF-κB signaling and other relevant genes involved in MF disease pathways (Mascarenhas J, et al. HemaSphere 2022;6:99-100). In the ongoing Phase 2 MANIFEST study (NCT02158858) (Kremyanskaya M, et al. Blood 2021;138:141) PELA is being evaluated as monotherapy in ruxolitinib (RUX)-intolerant, ineligible or refractory patients (pts) with MF (Arm 1) and in combination with RUX as an 'add-on' in pts with MF with suboptimal response to RUX (Arm 2) or in JAK inhibitor (JAKi)-naïve pts with MF (Arm 3). Assessment of ≥35% spleen volume reduction (SVR35) and ≥50% total symptom score reduction (TSS50) are standard clinical trial endpoints to evaluate disease response in MF. However, SVR35 and TSS50 do not fully reflect underlying disease improvement, and additional approaches are needed to understand what constitutes disease modification in MF (Pemmaraju N, et al. Cancer 2022;128:2420-2432) and to provide better insight into the mechanisms of novel drugs in development, such as PELA. Here we report results in pts enrolled in MANIFEST from exploratory analyses of treatment-induced changes at Week 24 in a range of biomarkers that are suggestive of disease modification (≥1 grade improvements in bone marrow fibrosis [BMF], reduction of Janus Kinase 2 V617F [JAK2V617F] variant allele frequency [VAF] in blood, improvements in hemoglobin and increased distance of CD61+ megakaryocytic [MK] cells in bone marrow ['declustering']) with respect to the clinical outcomes of SVR35 and TSS50. We previously demonstrated (Mascarenhas J, et al. HemaSphere 2022;6:99-100) that the increase in distance in MK cells correlated with SVR35 responses.
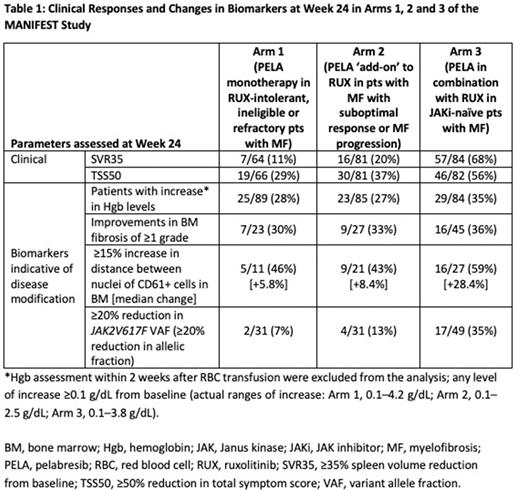
Table 1 shows a summary of SVR35 and TSS50 response rates and changes in biomarkers associated with potential disease modification observed in the MANIFEST study in each study arm. Of note, with PELA monotherapy, 30% (7/23) of pts in a RUX-resistant/intolerant population (Arm 1) showed ≥1 grade improvement in BMF. Additional changes indicating biologic effect of PELA included declustering of MK cells (increase in distance of ≥15%) observed in 46% (5/11) of pts. Similar or better results were observed in pts treated with PELA/RUX combination.
Changes in these markers of potential disease modification were associated with SVR35 and TSS50 endpoint responses. In evaluable pts with available data, splenic responses at Week 24 across the three arms were associated with ≥1 grade reduced BMF (in 18/43 pts, 42%), increased MK distance (in 20/26 pts, 77%, median change +34%) and JAK2V617F VAF reductions (in 41/50 pts, 82%, median change -13%). Of TSS50 responders at Week 24 with available data, 36% (16/44) showed ≥1 grade improvements in BMF; 71% (20/28) had an increase in MK distance (median change +22.3%); and 64% achieved reductions in JAK2V617F VAF (30/47, median change -8%). In JAKi-naïve pts (Arm 3), 68% (57/84) achieved SVR35 at Week 24; among these patients with available data, 38% (12/32) improved BMF ≥1 grade; 74% (14/19, median change +19.7%) increased MK distance; and 83% (34/41, median change -18%) reduced JAK2V617F VAF (any level of improvement). TSS50 was observed in 56% (46/82) of JAKi-naïve pts; among these patients with available data, improvements in ≥1 grade BMF, MK distance and JAK2V617F VAF (any level of improvement) were observed in 46% (11/24), 75% (12/16, median change +24%) and 78% (18/23, median change -14.3%) of pts, respectively. In nonresponders, changes in biomarkers were consistently smaller.
Our data indicate differences in the association of individual markers of potential disease modification with SVR35 and TSS50. While reduction in JAK2V617F VAF and increase in MK distance appear to be associated with SVR35, a high incidence of BMF improvements were also observed in Arm 3 TSS50 responders. Extended multivariable, longitudinal, biomarker analyses for association with time-to-event endpoints (eg duration of splenic response) will be presented with a new data cutoff with the objective to investigate potential disease-modifying activity of PELA.
Disclosures
Scandura:European Leukemia net: Honoraria, Other: Travel fees; CR&T: Research Funding; MPN-RF: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sumitomo Pharma Oncology, Inc: Consultancy; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Research Funding. Verstovsek:Genentech: Research Funding; CTI BioPharma Corp.: Research Funding; NS Pharma: Research Funding; Celgene: Consultancy, Research Funding; Roche: Research Funding; PharmaEssentia: Research Funding; Promedior: Research Funding; Protagonist Therapeutics: Research Funding; Sierra Oncology: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Gilead: Research Funding; ItalPharma: Research Funding; Incyte: Consultancy, Research Funding; Constellation Pharmaceuticals: Consultancy; Blueprints Medicines Corp.: Research Funding; AstraZeneca: Research Funding; Pragmatist: Consultancy. Kremyanskaya:Protagonist Therapeutics: Consultancy, Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; Chimerix: Research Funding; Incyte: Consultancy, Research Funding; Kronos: Research Funding; Kura: Research Funding; Ionis: Research Funding; BMS: Research Funding. Talpaz:SDp: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Grant/research support ; Kirin: Membership on an entity's Board of Directors or advisory committees; IMAGO: Consultancy; BMS: Consultancy; Novartis: Consultancy, Other: Grant/research support . Rampal:PharmaEssentia: Consultancy; Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; Stemline: Consultancy, Research Funding; Blueprint: Consultancy; CTI: Consultancy; Promedior: Consultancy; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Stemline: Consultancy, Research Funding; Zentalis: Consultancy, Research Funding; Sunimoto Dainippon: Consultancy; Jazz Pharmaceuticals: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy; Disc Medicines: Consultancy; Gilead: Consultancy; Novartis: Consultancy; Celgene/BMS: Consultancy; Incyte: Consultancy, Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding. Vannucchi:AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Morphosys: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Geron: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Gupta:Pfizer: Consultancy, Other: Participation on a Data Safety or Advisory board; Novartis: Consultancy, Honoraria; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Honoraria; AbbVie: Consultancy, Other: Participation on a Data Safety or Advisory board; BMS Celgene: Consultancy, Honoraria, Other: Participation on a Data Safety or Advisory board; Sierra Oncology: Consultancy; Roche: Other: Participation on a Data Safety or Advisory board. Palandri:Celgene: Consultancy, Honoraria; AOP: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Sierra Oncology: Consultancy, Honoraria; CTI: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Sobi: Consultancy, Honoraria; Kartos/Telios: Consultancy, Honoraria. Patriarca:Incyte: Honoraria, Speakers Bureau; Sanofi: Honoraria; Novartis: Honoraria, Speakers Bureau. Bose:Cogent: Honoraria, Research Funding; Ionis: Research Funding; Kartos: Research Funding; Promedior: Research Funding; Novartis: Honoraria; NS Pharma: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Honoraria, Research Funding; AbbVie: Consultancy; Sierra Oncology (now GSK): Consultancy; Pharma Essentia: Honoraria; CTI BioPharma: Honoraria, Research Funding; Disc Medicine: Research Funding; Telios: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; Karyopharm: Consultancy; BMS: Consultancy, Research Funding; Blueprint Medicines Corporation: Honoraria, Research Funding; Incyte: Honoraria, Research Funding. Zavidij:Constellation Pharmaceuticals, Inc., a MorphoSys Company: Ended employment in the past 24 months. Cui:Constellation Pharmaceuticals, Inc., a MorphoSys Company: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Chang:Constellation Pharmaceuticals, Inc., a MorphoSys Company: Current Employment. Taverna:Constellation Pharmaceuticals, Inc., a MorphoSys Company: Current Employment; Viracta Therapeutics: Current equity holder in publicly-traded company. Mascarenhas:Janseen: Research Funding; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Research Funding; Sierra Oncology: Consultancy; Galecto: Consultancy; Forbius: Research Funding; Merck: Research Funding; Kartos: Consultancy, Research Funding; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Research Funding; Prelude Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merus: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy; GSK: Consultancy; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imago: Consultancy; Roche: Consultancy, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Harrison:MPN voice: Other: Leadership role; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding; Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; EHA: Other: Leadership role; AOP Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Promedior: Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees; Sierra: Honoraria; Galacteo: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal